Servicios al cliente
Sobre nosotros
Copyright © 2025 Desertcart Holdings Limited


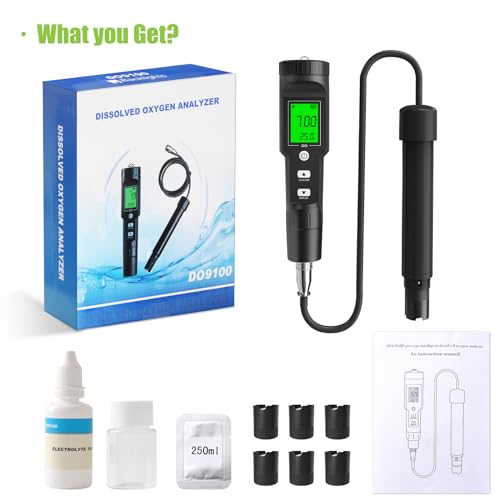
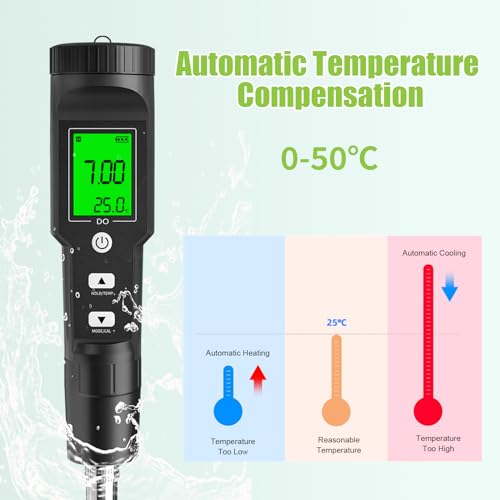

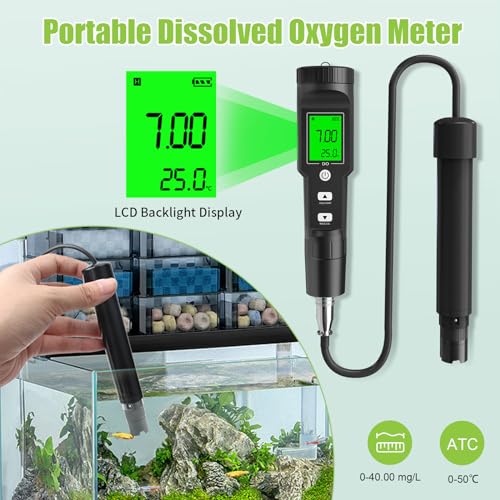

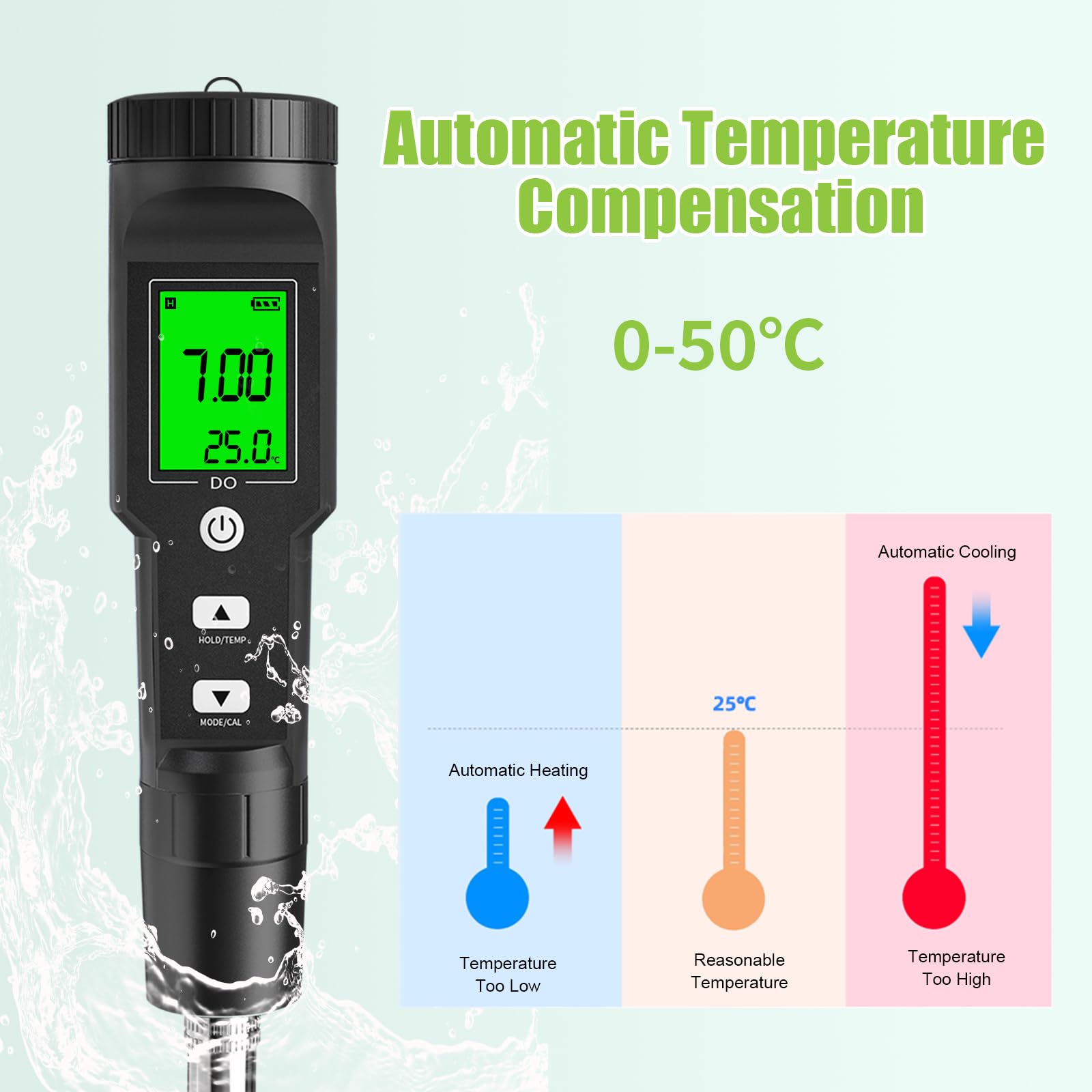


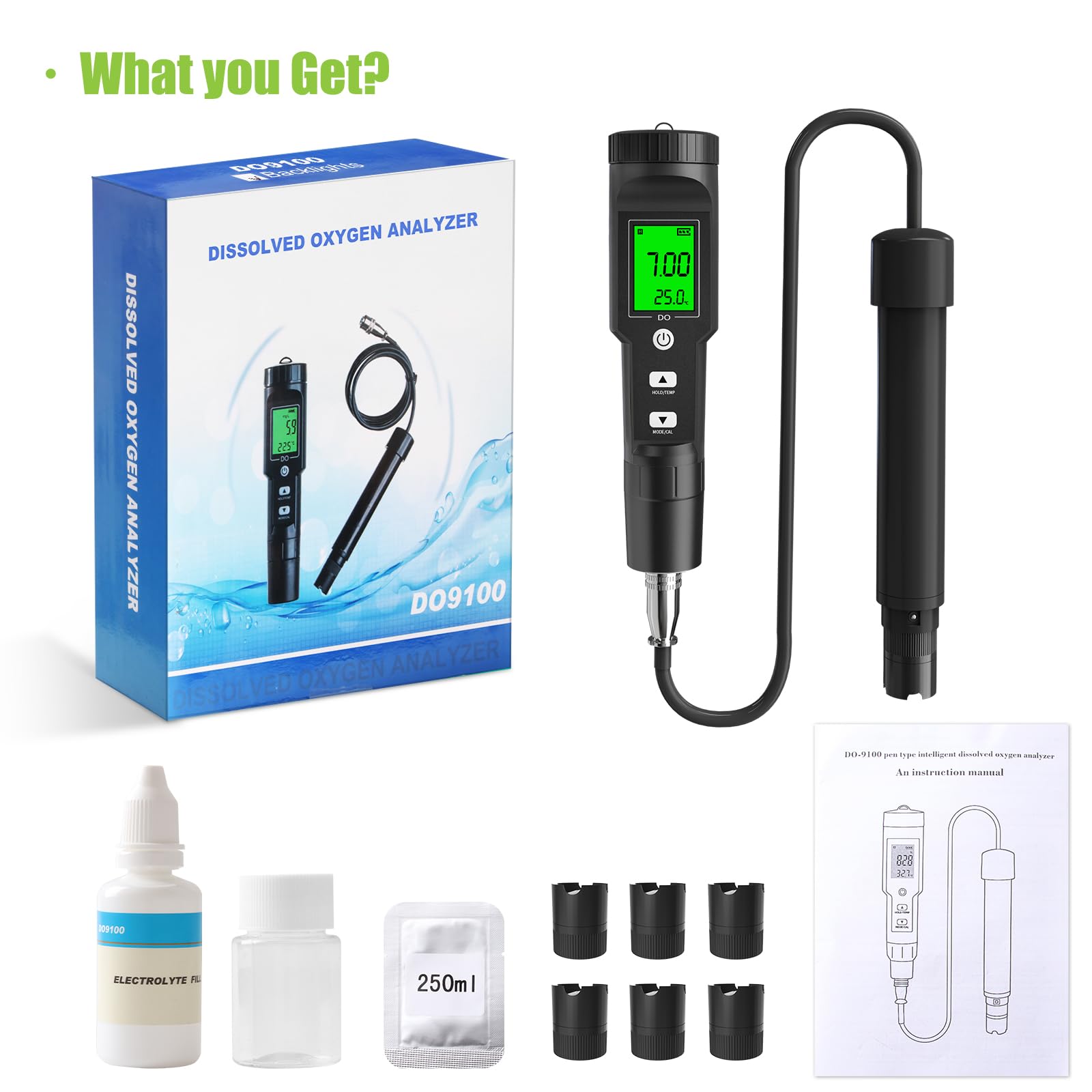


🌊 Dive into precision—because your aquatic life deserves the best!
The GISNPA Dissolved Oxygen Meter delivers lab-grade accuracy (±0.5mg/L) with a wide measurement range (0-40 mg/L) and smart auto temperature compensation (32-104°F). Designed for professional and hobbyist use alike, it features a large backlit LCD for easy reading and comes complete with electrode filling fluid, multiple probe covers, and anaerobic powder. Ideal for aquariums, ponds, aquaculture, and environmental testing, this compact, battery-included device ensures reliable, instant oxygen level monitoring to safeguard aquatic ecosystems.

| ASIN | B0CMXB81K4 |
| Are batteries included? | Yes |
| Best Sellers Rank | #16,010 in Industrial & Scientific ( See Top 100 in Industrial & Scientific ) #5 in Dissolved Oxygen Meters & Accessories |
| Brand | GISNPA |
| Country of Origin | USA |
| Customer Reviews | 3.4 3.4 out of 5 stars (34) |
| Date First Available | 19 December 2023 |
| Included Components | 6 Probe Covers, 1 Electrode Filling Solution, 1 Anaerobic Water Powder, 1 Manual |
| Item Dimensions LxWxH | 15.7 x 3.3 x 3.3 Centimeters |
| Item Height | 1.3 Inches |
| Item Weight | 390 g |
| Item Width | 1.3 Inches |
| Item part number | DO9100-A |
| Manufacturer | GISNPA |
| Number of Memory Sticks | 1 |
| Product Dimensions | 15.75 x 3.3 x 3.3 cm; 390 g |
S**Y
This GISNPA Dissolved Oxygen meter is a good, basic meter. It comes with electrolyte filling solution and 6 additional membranes, which is more than enough to last for quite a bit of use. I'll be using this to monitor a deep well to watch for casing cracks. Calibration in 100% air mode is quick and easy. Temperature quickly switches between deg C and deg F, and % oxygen or ppm is equally easy to change at the press of a button. A quick check of tap water provided a reasonable result, 5 minutes after opening the box. I've worked with laboratory DO meters for my entire career in chemistry and, for personal use, this meter does the job just as well as professional bench-top models. For those who couldn't keep a good calibration, try putting some water in the membrane cap (not where the electrolyte goes, but the protective cap instead). Bench-top meters usually have a DO bottle with water that you store the probe in, so storing a probe dry is the worst thing for the membrane. It would be nice if the mfg. would provide a link for additional filling solution, calibration powder, and membranes. Also, they should provide a Safety Data Sheet (SDS) to be fully compliant with OSHA regulations. Without the SDS, there is no safety information for accidental ingestion (it happens, with pets and kids in a household. Better safe than sorry).
J**N
El producto llego un día antes de lo esperado, que fue excelente porque ya lo necesitabamos. El equipo funciona bien y es fácil de manejar.
L**T
Entire wand must be immersed to accurately capture temperature of specimen. Will not return correct DO results if not fully into sample.
E**E
Stopped working after about 3 months
L**U
I got this to check the dissolved O2 in the water supply for my indoor garden. As water sits, the air which is dissolved in it will slowly escape from the surface of the water. Water which is devoid of dissolved oxygen (among other things) is NOT good for plants. Think about where plants get their water. Rain. What happens when rain falls? It dissolves a LOT of air on the way down. So the water plants normally get in nature is filled with dissolved O2. If you have a tub of water just sitting there, your watering system will be pumping dead, O2-free water to your plants because most of the air will have escaped. I run an air bubbling system through my water supply to keep it aerated. This tool allows me to see just how much (or how little) air I'm putting into my water supply, which in turn tells me if I need to add more air or if perhaps I can dial it back a bit to save energy. The instructions are pretty horrible, so I'll break down the setup process for you here. This comes with a bottle of sodium sulfite solution. You unscrew the membrane cap from the probe, and fill it up until around where the threads end on the inside. Now here is the critical step that will determine how accurate it is or isn't. Once you have it full to the proper point, you need to look down into the cap and see if there are ANY tiny little bubbles sticking around down near the bottom, they will tend to collect where the membrane meets the cap body. You need to gently tap around the cap, holding it upright of course, to get every one of those little bubbles to come out. Some will be VERY small and will require some persistence to get them dislodged. I like to use a small tapping tool instead of my finger, but your finger will work well. I find a glass stir rod from any chemistry set works well, as does the handle of a teaspoon or a chopstick. The important thing is to have ALL the bubbles gone, and when they're gone make sure they've popped at the top by still MORE tapping. Once every last bubble is gone, gently screw the cap onto the probe. You should have put enough sodium sulfite solution (the stuff in the little white bottle) that when you screw the cap on, you get a few drops spill out when you screw it all the way down. Just wipe them up, there is nothing hazardous about sodium sulfite. From an actual sodium sulfite MSDS: "This chemical is not considered hazardous by the 2012 OSHA Hazard Communication Standard (29 CFR 1910.1200)". So there is nothing in the solution to hurt you, just wipe it with a paper towel and throw it away. The slight overflow will ensure that no bubbles are present in the probe solution. Bubbles will directly impact accuracy. Turn it on and let it sit for a bit, run an open air cal on it and you should be good to go. They include a nice sample bottle that fits directly over the probe end. Put your sample in there and gently rock it back and forth to keep the water moving, but not so hard that you splash the water, which will cause the reading to go high. Once you have a reading, you can actually shake the bottle harder, and watch the dissolved O2 levels rise, and then let it sit, and you can watch them fall as the O2 escapes away again. This is a good indicator the tester is working properly, if you can watch static levels fall and agitated levels rise, because this is exactly what is happening when you shake water. I like the overall design of the tester, though mostly plastic it seems to be a decent quality plastic, and you get 6 replacement probes for when they wear out or become damaged. I am unaware whether or not replacements are available beyond that. The sodium sulfite solution should be adequate for quite a few fills, but if you run out, sodium sulfite is ridiculously cheap and widely available. You can get 1 lb of lab-grade sodium sulfite here on Amazon for less than $20 which you can make your own solution with. You can also make sodium sulfite any number of ways for next to nothing, such as burning sulfur to create sulfur dioxide and then bubbling sulfur dioxide through sodium hydroxide solution. Once again, super cheap to make. It's a great tool to have and use if you set it up and use it properly. As I said earlier, the filling of the probe cap is the critical part, and if you do this right, all is good. The only reason I took a star off is because it's powered by LR44 / 357 button-cell batteries. 3 of them. I really would prefer it this was rechargeable. I mean, how prevalent are compact LiPo packs? But no, LR44 batteries. That's my only gripe with this, is the power source. If you buy this, my advice is pick up some extra 357 batteries (3 of them) so you have spares on hand. A handy tool, for sure, it worked well and seemed to be accurate, without a similar tool to test it against. Recommended. If they come out with a rechargeable version? I'd recommend it even more.
Trustpilot
Hace 1 mes
Hace 3 días